
A short link is a powerful marketing tool when you use it carefully. It is not just a link but a medium between your customer and their destination. A short link allows you to collect so much data about your customers and their behaviors.
Target your customers to increase your reach and redirect them to a relevant page. Add a pixel to retarget them in your social media ad campaign to capture them.
Share your links to your network and measure data to optimize your marketing campaign's performance. Reach an audience that fits your needs.
Use various powerful tools increase conversion and provide a non-intrusive experience to your customers without disengaging them.
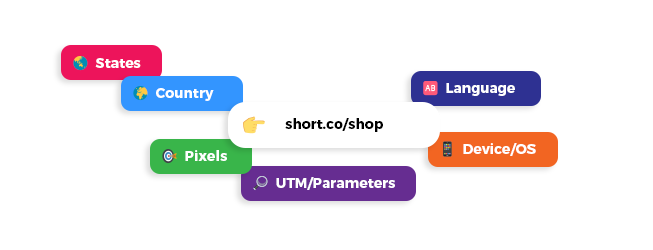
Understanding your users and customers will help you increase your conversion. Our system allows you to track everything. Whether it is the amount of clicks, the country or the referrer, the data is there for you to analyze it.

Our product lets your target your users to better understand their behavior and provide them a better overall experience through smart re-targeting. We provide you many powerful tools to reach them better.

Easy to use, dynamic and customizable QR codes for your marketing campaigns. Analyze statistics and optimize your marketing strategy and increase engagement.
Get Started


Understanding your users and customers will help you increase your conversion. Our system allows you to track everything. Whether it is the amount of clicks, the country or the referrer, the data is there for you to analyze it.
Get StartedCreate a custom landing page to promote your product or service on forefront and engage the user in your marketing campaign.
Use our overlay tool to display unobtrusive notifications, polls or even a contact on the target website. Great for campaigns.
Add your custom pixel from providers such as Facebook and track events right when they are happening.
Invite your team members and assign them specific privileges to manage links, bundles, pages and other features.
Easily add your own domain name for short your links and take control of your brand name and your users' trust.
Use our powerful API to build custom applications or extend your own application with our powerful tools.
Connect with popular tools and boost your productivity.